Abstract

Background: Clonal cytopenia(s) of undetermined significance (CCUS) is defined as persistent cytopenias arising in the context of myeloid neoplasm (MN)-associated somatic mutations (MT) in hematopoietic stem cells. Patients (PTs) with TET2 mutant (TET2MT) CCUS have a high probability of progression to MN. To date, no FDA-approved treatment for CCUS exist, with PTs often having similar cytopenias and transfusion needs as those with myelodysplastic syndromes (MDS). High dose intravenous ascorbic acid (HI-AA) demonstrates anti-cancer activity via two mechanisms: (1) hydrogen peroxide-induced oxidative stress and (2) DNA demethylation mediated by TET activation (cofactor for TET1/2/3). TET2 is an AA and oxoglutarate-dependent dioxygenase that catalyzes the conversion of 5-methylcytosine to 5-hydroxymethylcytosine (5hmC), providing the rationale to study HI-AA in TET2MT CCUS.
Methods: This is an investigator-initiated, single-arm, single-institution, phase II trial assessing the safety and preliminary efficacy of HI-AA for PTs with TET2MT CCUS (NCT03418038). PTs³ 18 years with TET2MT CCUS (≥1 TET2MT with or without additional somatic MTs) and with any of the following laboratory criteria: (1) hemoglobin (Hb) ≤10g/dL, (2) absolute neutrophil count (ANC) ≤1(10^9/L), (3) platelet count (PLT)≤100 (10^9/L) are eligible. HI-AA (1g/kg, maximum 100g) is given 3 times weekly for 12 weeks through a PICC line, with response assessments before each cycle (4 weeks), at 20 weeks, and one year. The primary endpoint is the hematologic response rates determined by the proposed MDS IWG 2018 criteria at week 20. (Platzbecker Blood 2018) and reported as hematologic improvement (HI)- erythropoietic (HI-E), platelets (HI-P), neutrophils (HI-N). Secondary endpoints include safety and adverse events (AEs). AEs were graded using NCI-CTCAE v4.03. Correlative studies include changes in TET2MT variant allele fraction (VAF), TET2 activity, DNA methylation, hydroxymethylation, and 5hmC in bone marrow cells as determined by immunohistochemistry (IHC).
Results: As of July 22, 2022, 8 (89%) of 9 enrolled PTs completed 12 weeks of therapy. The mean age was 74 (SD: 5.4) years, 7 (87.5%) males. One PT (PT_X) came off study after cycle one due to a PICC line-associated thrombosis. All PT were TET2MT (44% truncating), with 8 of 9 (89%) having co-mutations (4- 2 MTs, 3- 3 MTs, and 1- 4 MTs; 2 had ≥1 TET2MT). Comutations included SRSF2 (55%), ASXL1 (22%), ZRSR2 and KRAS (11% each). HI-AA was well tolerated with the most common AEs being infusion-related polyuria (56%) and polydipsia (44%, 3 Grade 1, 1 Grade 2). One (11%) PT had constipation and headaches (both Grade 1), and 1 (11%) experienced dyspepsia. No treatment-related Grade 3 or 4 AEs or deaths were observed.
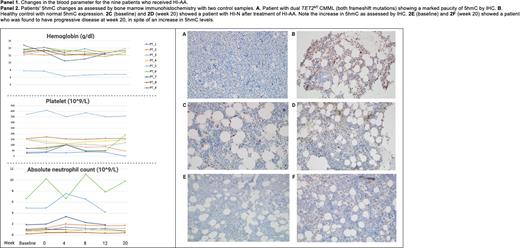
Six of 8 (75%) assessable PTs had post-treatment response assessments at week 20, with 3 PTs having completed 1-year assessments. All PTs' baseline Hb was within the normal range, except for 1 (17%) (PT_5- TET2MT/SRSF2MT) PT who continued to remain red blood transfusion dependent. Five (63%) PTs were thrombocytopenic PLT <100 (10^9/L), with 2 (25%) PTs (PT_1 and PT_7) having PLT £50 (10^9/L) and 5 (83%) PTs had ANC<1 (10^9/L) with 1 (17%) (PT_8) with ANC <0.5 (10^9/L). One (17%) PT met criteria for HI-P (PT_6 had an increment to 118 (10^9/L) at week 20- TET2MT/SRSF2MT/KRASMT) and 1 (17%) PT met criteria for HI-N (PT_2, ANC baseline:1.08 vs. week 20: 1.76(10^9/L)-TET2MT/SRSF2MT). PT_1, who had a baseline PLT of 25 (10^9/L) and ANC of 0.72 (10^9/L), had disease progression to MDS-EB-1 (TET2MT/SRSF2MT) at week 20. The 3 PTs with 1 year follow-up have stable disease. Panel 1 demonstrates changes in blood parameters with treatment. Panel 2 shows IHC determined changes in 5hmC changes with two control samples: normal control and TET2MT CMML. In 5 assessable PTs, there were no changes in TET2 VAF at 20 weeks (mean VAF : baseline 37.2% vs. week 20: 36.6%, p=0.3). Additional correlative studies are ongoing.
Conclusion: We report safety and preliminary efficacy analysis for the first clinical trial dedicated to TET2MT CCUS. The early results of this pilot demonstrate that HI-AA is safe and has potential for biological and clinical effects. At last follow-up, 2 (33%) PTs met criteria for HI (HI-P-1 and HI-N-1), 3 PTs (50%) had stable disease, and 1 (17%) PT progressed to MDS. Future studies assessing potential synergy of adding HI-AA to standard of care therapies for TET2MT clonal processes are planned.
Disclosures
Mangaonkar:Bristol Myers Squibb: Research Funding. Witzig:Kura Oncology: Other: Clinical Trail Support; Curio Science: Honoraria; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Other: Clinical Trail Support. Patnaik:Kura Oncology, Stemline Therapeutics: Research Funding.
OffLabel Disclosure:
Drug: Ascorbic acid Purpose: To improve cytopenia(s) in patients with TET2 mutant clonal cytopenia
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal